Roeffaers Lab
Nanoscopy and catalysis
Equipment
Equipment available in the Roeffaers-Lab:
In house we developed an integrated fluorescence and electron microscope that combines a field emission scanning electron microscope with a wide-field fluorescence microscope capable of detecting individual fluorescent molecules. This unique setup allows us to perform highly detailed structural imaging with the electron microscope and the functionality of the material can simultaneously or consecutively be captured at the smallest possible length scales using fluorescence microscopy. When investigating solid acid catalysts for example, structural information can be correlated with highly resolved activity maps at the nanoscale (NASCA microscopy). In an alternative application the optical part of the setup can be applied to activate photocatalyst particles by means of UV irradiation while the particle structure can be monitored at the same time.
Details on the setup:
The integrated instrument is based on a FEI Quanta 250 FEG environmental scanning electron microscope, equipped with an EDX detector (129eV, 60mm Octane Silicon Drift Detector) and the SECOM platform acquired from Delmic BV. An Omicron laserhub provides laser excitation with 6 different wavelengths (405, 445, 488, 532, 561 and 642 nm) and 365 nm UV light can be provided by an additional LED source (Thorlabs). The excitation light is transmitted into the sample chamber through an optically transparent window in the SEM door and directed onto the sample by a high numerical aperture oil-immersion objective lens (Nikon plan APO VC 100x, 1.4 NA). In combination with vacuum compatible immersion oil this enables high-resolution imaging. The emitted fluorescence signal is captured by the EMCCD camera (Hamamatsu C9100-23B) positioned on the outside of the SEM door. The optical pathway in our specific system differs from the standard SECOM platform, as it is in house designed to allow maximum flexibility. It is e.g. possible to introduce additional optical elements to enable polarized light experiments. Finally, the availability of a liquid sample holder that combines an optically transparent cover slide on the one side and an electron transparent silicon nitride window on the other, enables truly integrated experiments.
Funding agencies:
ERC (LIGHT GA-307523)
Hercules (HER/11/14)
FWO (G.0962.13)
Related scientific publications:
Debroye, E.; Van Loon, J.; Gu, X.; Franklin, T.; Hofkens, J.; Janssen, K. P. F.; Roeffaers, M. B. J. Assessing Photocatalytic Activity at the Nanoscale Using Integrated Optical and Electron Microscopy. Part. Syst. Charact. 2016, 33, 412–418.
The fundamental vibrational signatures of molecules can report on their interaction with the local environment. This way, the use of specific probe molecules in vibrational spectroscopy has significantly contributed to the understanding and further development of catalysts. The spatial resolution of spontaneous Raman microscopy is comparable to that of fluorescence microscopy, since light of similar wavelengths is used. However, its sensitivity is much lower because of its 1012 times lower cross-section. Such weak Raman signals can be enhanced by nonlinear excitation, i.e. Coherent Anti-Stokes Raman scattering (CARS) or Stimulated Raman scattering (SRS). Instead of using one laser, a pump (ωp) and Stokes (ωs) laser are used. When the difference in wavelength between these two lasers matches the wavelength of a molecular vibration (ωp - ωs = Ω), the coherent generation at the Anti-Stokes frequency (2ωp - ωs) will be greatly enhanced. CARS is easily detected since it is generated at a different frequency as the pump and Stokes photons. A drawback of this technique is the inherent non-resonant background which can make spectrum interpretation/assignment difficult. Under the same conditions as CARS, also stimulated excitation takes place, i.e. Stimulated Raman scattering (SRS); pump photons are converted more rapidly into additional Stokes photons. As a result, the Stokes beam gains intensity (= stimulated Raman gain, SRG) and the pump beam loses intensity (= stimulated Raman loss, SRL). Since the SRG and SRL are generated at the same frequency as the two input laser beams, a high-frequency amplitude modulation is needed for detection. Unlike CARS, SRL and SRG do not exhibit a non-resonant background. Both techniques have a single frequency approach, since the condition of "ωp - ωs = Ω" needs to be fulfilled, and therefore, a tunable laser or an OPO is needed in order to study a sample at different wavelengths.
Both techniques have a wide array of applications, ranging from label-free bio(medical) research to materials science. In our group, we mainly apply probe molecule mediated micro(spectro)scopy for materials research. One of the applications is the investigation of single zeolite catalyst particles. By using the correct Raman probe molecule (e.g. nitriles or pyridines), the strength, accessibility and type of acid sites inside these zeolite particles can be determined. The submicron resolution and video-rate imaging speeds even allow diffusion inside these zeolites to be investigated. Besides solid catalysts, also geological samples are being investigated and Coherent Raman and multiphoton processes are applied to look deeper into biological questions, such as the determination of the lipid content in living organisms and the visualization of collagen scaffolds in tissues and hydrogels.
Details on the setup:
The Coherent Raman setup uses the second harmonic output (at 532 nm) of a Nd:YVO4 laser (picoTRAIN, High-Q) to pump an optical parametric oscillator (Levante Emerald, APE Berlin). This laser has a repetition rate of 80 MHz and 7 ps pulses. The OPO employs a temperature-tuned noncritically phase-matched LBO crystal and an intracavity Lyot filter to allow continuous tuning of the OPO output. The tunable pump beam (700 - 980 nm) is used along with the 1064 nm Stokes beam to probe molecular vibration. These are then combined using a dichroic mirror and guided into the microscope. The generated Coherent Anti-Stokes Raman scattering is collected both in the forward and epi-direction. For detection of Stimulated Raman scattering, the Stokes beam is amplitude-modulated at 9.7 MHz with a Pockel Cell (model 360-80, ConOptics) triggered by a function generator (model 29, Waveteck). After collection of the light by the condenser, the Stokes beam gets filtered out by a bandpass filter. The stimulated Raman loss (SRL) of the pump beam is then detected by a large-area silicon PIN photodiode (S8650, Hamamatsu) with a reversed bias of 60 V. The output photocurrent is low-pass-filtered (Mini-Circuits, BLP-10.7) and demodulated by a lock-in amplifier (HF2LI, Zurich Instrument). The output of the lock-in amplifier is fed into the analog-to-digital converter (FV-10-ANALOG, Olympus) and synchronized with the scanning unit. We also employ a femtosecond multiphoton laser to excite two-photon transitions or to perform second harmonic generation experiments (SHG). The laser (Maitai Deepsee Series of Spectra Physics) is tunable between 650 and 1050 nm with a nominal pulse duration of 150 fs. An Olympus FV1000 microscope is used for scanning all laser beams. Fluorescence and SHG photons are detected using photomultipliers.
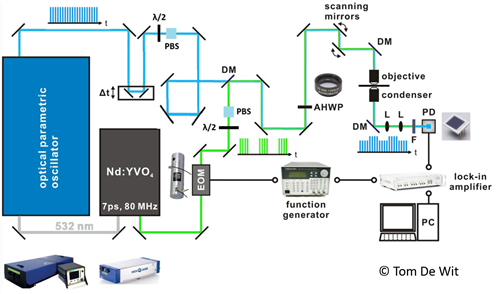
Figure: Schematic diagram of the Coherent Raman setup showing the various components and the Nd:YVO4 laser. EOM : Electro-optic modulator, Δt: Delay stage, λ/2: Half-wave plate, PBS: Polarizing beam splitter, DM: Dichroic mirror, AHWP: Achromatic half wave plate.
Related scientific publications:
Liu, K. L.; Kubarev, A. V.; Van Loon, J.; Uji-I, H.; De Vos, D. E.; Hofkens, J.; Roeffaers, M. B. J. Rationalizing Inter- and Intracrystal Heterogeneities in Dealuminated Acid Mordenite Zeolites by Stimulated Raman Scattering Microscopy Correlated with Super-Resolution Fluorescence Microscopy. ACS Nano 2014, 8, 12650–12659.
The fingerprinting of Raman scattering signals from different materials (gases, liquids and solids) has become a powerful nondestructive structural characterization tool for materials research. The custom and dynamic design of the micro-Raman scattering instrument housed at the Roeffaers lab, permits a wide range of Raman studies for different purposes. The kind of structural information that can be acquired with our custom built instrument extends beyond standard Raman spectral fingerprinting; for example, topological structural/compositional characterization is achieved through high resolution surface mapping, and molecular orientation and bonding symmetry/order (from crystalline to amorphous phases) can be determined via polarization dependence measurements. Combined, the range of dynamic elements integrated into our Raman microscope secures it as a genuine research-grade instrument, delivering unmatched performance and flexibility.
Details on the setup:
The Raman (micro)spectroscopy setup is an in-house design and has different laser light sources (Argon, HeNe gas and UV solid state laser), as well as frequency doubling media (BBO crystals). As such, this instrument allows excitation with one of multiple laser lines, covering the deep UV and VIS (244, 257, 375, 458, 488, 514 and 633 nm) wavelengths. The incident and scattered laser light is coupled into an inverted Olympus IX71 microscope, equipped with a set of high NA oil immersion objective lenses (60X, 100X - Olympus and Zeiss), low NA air objectives (x10, x20 - Olympus) and a deep UV objective (40X – Thorlabs). A motorized XYZ stage (Märzhäuser Wetzlar) enables precise sample manipulation and Raman surface mapping of materials, which can be visualized by means of a deep UV-extended camera (Allied Vision Prosilica GE1650). All optical elements of the microscope (cameras, mirrors, beam splitters, lenses, polarizing optics, etc.) can be adapted to facilitate high optical throughput and full functionality for wavelengths covering deep UV to VIS wavelengths. Backscattered optical signals are sensitively detected using a liquid nitrogen-cooled CCD camera, which is also capable of performing time resolved studies with a good temporal resolution. Finally, a TriVista triple spectrometer setup (Princeton Instruments) is included into the setup. As it operates as three individual monochromators, or as combinations of single & double spectrometers, it allows an excellent spectral resolution to be achieved over the entire dispersion range, as well as extreme stray light rejection. This enables Raman spectra to be captured as close as ~40 wavenumbers from the Rayleigh laser line.
Light sheet fluorescence microscopy (LSFM) provides a solution to the trade-off between imaging speed and photobleaching for scanning large volumes at decent resolutions. In LSFM, a dedicated illumination system accomplishes simultaneous fluorescence excitation along a plane in the sample. Imaging of the generated fluorescence signals is achieved through a lens positioned under a 90-degree angle, hence, only fluorescence coming from a zone with a restricted depth is observed, which drastically improves the obtained contrast. Furthermore, by using long working distance, high numerical aperture lenses with color and image aberration correction, it is possible to image larger samples in 3D. So far the use of LSFM was mainly restricted to the field of developmental biology as a means to investigate embryonic development in a dynamic fashion. Its application in material sciences is still unexplored. Therefore, we have extended the use of LSFM in the Roeffaers group to:
1) investigate cell-matrix mechanics in three dimensions. These interactions play an important role in biological processes like angiogenesis, i.e. the regeneration and development of new blood vessels from existing vasculature.
2) study heterogeneous catalysts. In industrial applications these catalyst particles are often shaped into larger composite catalyst bodies using binder material. However, a detailed visualization of the effect and contribution of this binder on the catalytic performance throughout the whole catalyst body is not possible with conventional epi-fluorescence microscopy.
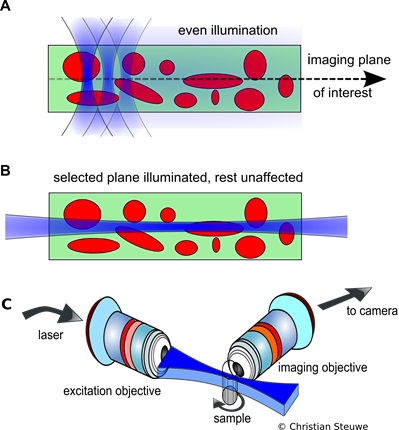 Figure A: In confocal scanning, a ROI is constantly being irradiated with in and out of focus light, which is overcome by light sheet imaging. B: Only a selected plane is illuminated which is then imaged onto a camera chip. This is more efficient and reduces bleaching. C: The setup configuration employed for light sheet imaging.
Figure A: In confocal scanning, a ROI is constantly being irradiated with in and out of focus light, which is overcome by light sheet imaging. B: Only a selected plane is illuminated which is then imaged onto a camera chip. This is more efficient and reduces bleaching. C: The setup configuration employed for light sheet imaging.
Details on the setup:
The whole setup is custom-made and highly adaptable. It includes a long working distance, high numerical aperture, objective lens (NA=0.4 or 0.8) with color and image aberration correction. Excitation is achieved at 532 nm, 488 nm, 375 nm and near infrared, by means of a pulsed femtosecond laser and dual channel detection is done using two Hamamatsu ImageEM EMCCD cameras.
The optical sectioning capabilities of confocal laser scanning microscopes (CLSM) makes for a very versatile and popular imaging tool in research. However, due to the diffraction limit of light these optical systems are unable to discern details that are closer together than half the wavelength of the light being emitted (Abbe’s limit)
Fluorescence nanoscopy encompasses a number of methods to break this diffraction limit and enables the direct study of structures at the nanometer scale. One of these nanoscopy techniques that is implemented on the system is STimulated Emission Depletion (STED). This technique uses two laser beams with temporal overlap, one to excite fluorophores at a diffraction limited spot and another one for selective deactivation of fluorophores in a ring around the focal spot. What’s left is a nanometer sized region in the center where fluorescence emission can still occur.
Details of the setup:
The Leica TCS SP8 system features a Leica DMi8 inverted microscope with a motorized stage and various objective lenses (10x to 100x). Besides a pulsed 405nm diode laser, the setup features a supercontinuum laser which allows continuously tunable excitation in the visible range coupled with filter-free detection. Two PMT detectors and four internal hybrid (HyD) detectors are available which offer high sensitivity and superior signal-to-noise ratio. The systems tandem scanner unites the standard scanner unit with a 8kHz resonant scanner enabling very fast acquisition. Temperature and CO2 control are available for live cell imaging. The systems Lambda square mapping ability allows full spectral analysis of samples and optimization of excitation and detection range.
Included modalities:
- STED nanoscopy: The SP8 STED 3x enables depletion with three different laser lines (592nm CW, 660nm CW, 775nm pulsed), allowing super-resolution imaging both in lateral and axial direction.
- 2-Photon microscopy: The setup is equipped with a tunable MaiTai DeepSee femtosecond laser (690nm - 1040nm) for 2-Photon excitation. Two non-descanned HyD detectors for optimal 2-Photon acquisition are included in the setup.
- Digital Lightsheet: With minimal adaptation the SP8 can be used as a lightsheet microscope, utilizing Leica’s TwinFlect mirror design for single plane illumination.
- TCSPC: The system is coupled with PicoQuant’s time-correlated single photon counting hardware and software enabling various time resolved techniques like FLIM, FRET and FCS.
Confocal laser scanning microscopy (CLSM) improves the resolution that is obtained in wide-field illumination techniques by incorporating a pinhole at the confocal plane of the lens. By doing so, out-of-focus light is eliminated, increasing the optical resolution (mainly along the depth of the sample) as well as the contrast. As such, this point-by-point imaging technique also allows optical sectioning of thick specimens, resulting in a better understanding of topologically complex materials by means of a three-dimensional reconstruction. In our group, this approach is used for materials research such as imaging of (fluorescent) organic molecules in single zeolite or metal-organic framework (MOF) particles to name a few. With this approach catalytic activity mapping or diffusion inside a crystal can be measured. Additionally, CLSM is applied in cell biology to investigate certain properties through the interaction with fluorescent molecules. To facilitate a large number of users, the lab is equipped with two individual CLSM setups, FV1000 from Olympus.
Details of the setup:
Both CLSM setups are motorized inverted system microscopes, Olympus IX81 (IX2 Series), with a motorized universal condenser which enables standard brightfield, phase contrast and differential interference contrast (DIC) observations. The Prior ProScanTM III stage has been included into the system to allow automation control over the microscope. An integrated laser combiner provides multiple excitation laser lines, ranging from UV to visible light (375, 405, 440, 445, 488, 515, 532, 559, 561, 635 nm). Various objective lenses (4X to 100X, NA: 0.16 to 1.4) can be utilized for image optimization, depending on the sample. Live cell imaging is also possible with the inclusion of an incubator, ensuring temperature just below 37 oC, working alongside with a CO2 flow control.

Related scientific publications:
Valvekens, P.; Jonckheere, D.; De Baerdemaeker, T.; Kubarev, A. V.; Vandichel, M.; Hemelsoet, K.; Waroquier, M.; Van Speybroeck, V.; Smolders, E.; Depla, D.; Roeffaers, M. B. J.; De Vos, D. Base Catalytic Activity of Alkaline Earth MOFs: a (Micro)spectroscopic Study of Active Site Formation by the Controlled Transformation of Structural Anions. Chem. Sci. 2014, 5, 4517–4524.
Wide-field fluorescence microscopy allows for fast, up to 30 frames per second, and sensitive, down to the level on individual fluorophores, detection. This kind of microscopy is used extensively in our group to investigate the properties of individual heterogeneous catalyst particles. Most common applications include the investigation of catalytic activity via probe fluorogenic reactions (NASCA microscopy) and adsorption and diffusion studies via the use of fluorescent dye molecules. By precise localization of individual chromophores the diffraction-limited resolution can be overcome and nanoscale pictures of the material under study can be recorded. The employed setups are built in-house and can be adapted for various types of experiments thanks to the possibility to introduce additional optical elements.
Details on the setup:
The setups are mainly based on inverted microscope bodies, Olympus IX71 and Olympus IX83, equipped with a set of oil immersion high NA objective lenses (60x, 100X, Olympus) and different laser wavelengths (405 nm, 448 nm, 491 nm, 514 nm, 532 nm, 561 nm, 640 nm). These lasers give a large flexibility regarding the types of chromophores that can be imaged. It is possible to combine 2 or more laser lines to achieve multicolor excitation. Additionally, the use of polarizing optics makes it possible to study molecular orientation inside complex materials. Fluorescence imaging is performed by highly sensitive EM-CCD cameras such as the ImagEM X2 C9100-23B EM-CCD camera from Hamamatsu. Also from the detection side multicolor detection is possible.
Funding agencies:
ERC (LIGHT GA-307523)
Hercules (HER/11/14)
FWO (G.0962.13)
Related scientific publications:
Z. Ristanović, A. V. Kubarev, J. Hofkens, M. B. J. Roeffaers and B. M. Weckhuysen, J. Am. Chem. Soc., 2016, 138, 13586–13596.
A. V. Kubarev, K. P. F. Janssen and M. B. J. Roeffaers, ChemCatChem, 2015, 7, 3646–3650.
Z. Ristanović, J. P. Hofmann, G. De Cremer, A. V. Kubarev, M. Rohnke, F. Meirer, J. Hofkens, M. B. J. Roeffaers and B. M. Weckhuysen, J. Am. Chem. Soc., 2015, 137, 6559–6568.
Z. Ristanović, M. M. Kerssens, A. V. Kubarev, F. C. Hendriks, P. Dedecker, J. Hofkens, M. B. J. Roeffaers and B. M. Weckhuysen, Angew. Chemie - Int. Ed., 2015, 54, 1836–1840.
K. L. Liu, A. V. Kubarev, J. Van Loon, H. Uji-I, D. E. De Vos, J. Hofkens and M. B. J. Roeffaers, ACS Nano, 2014, 8, 12650–12659.
P. Valvekens, D. Jonckheere, T. De Baerdemaeker, a. V. Kubarev, M. Vandichel, K. Hemelsoet, M. Waroquier, V. Van Speybroeck, E. Smolders, D. Depla, M. B. J. Roeffaers and D. De Vos, Chem. Sci., 2014, 5, 4517–4524.