Roeffaers Lab
Nanoscopy and catalysis
ACS Catalysis publication on H-mordenite catalysis
Article Published in ACS Catalysis
Rationalizing Acid Zeolite Performance on the Nanoscale by Correlative Fluorescence and Electron Microscopy
Abstract
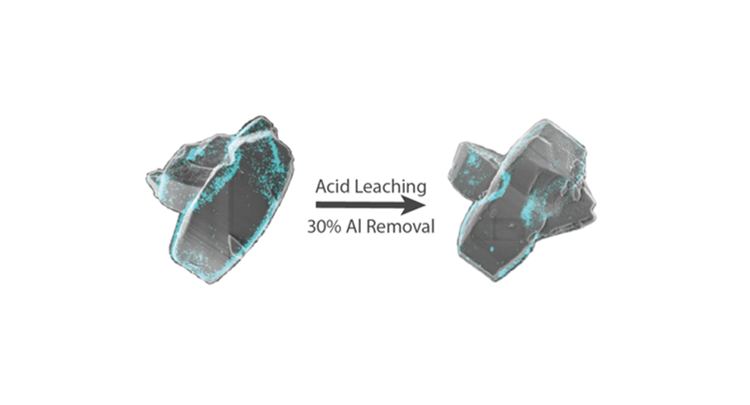
The performance of zeolites as solid acid catalysts is strongly influenced by the accessibility of active sites. However, synthetic zeolites typically grow as complex aggregates of small nanocrystallites rather than perfect single crystals. The structural complexity must therefore play a decisive role in zeolite catalyst applicability. Traditional tools for the characterization of heterogeneous catalysts are unable to directly relate nanometer-scale structural properties to the corresponding catalytic performance. In this work, an innovative correlative super-resolution fluorescence and scanning electron microscope is applied, and the appropriate analysis procedures are developed to investigate the effect of small-port H-mordenite (H-MOR) morphology on the catalytic performance, along with the effects of extensive acid leaching. These correlative measurements revealed catalytic activity at the interface between intergrown H-MOR crystallites that was assumed inaccessible, without compromising the shape selective properties. Furthermore, it was found that extensive acid leaching led to an etching of the originally accessible microporous structure, rather than the formation of an extended mesoporous structure. The associated transition of small-port to large-port H-MOR therefore did not render the full catalyst particle functional for catalysis. The applied characterization technique allows a straightforward investigation of the zeolite structure–activity relationship beyond the single-particle level. We conclude that such information will ultimately lead to an accurate understanding of the relationship between the bulk scale catalyst behavior and the nanoscale structural features, enabling a rationalization of catalyst design.
DOI: 10.1021/acscatal.7b01148
To enable comments sign up for a Disqus account and enter your Disqus shortname in the Articulate node settings.