Roeffaers Lab
Nanoscopy and catalysis
PhD defence
PHD Defence Wouter Vandezande

Volatile organic compounds (VOCs) are chemicals found in different places like homes and industries, coming from both nature and human activities. Since VOCs can be harmful to health and the environment, it's important to find and keep track of them. The usual way to do this is with an expensive method called gas chromatography with mass spectrometry, but it cannot provide real-time, on-site measurements.
To solve this problem, this Ph.D. project worked on making a sensor that is both affordable and versatile. Fiber-optic surface plasmon resonance (FO-SPR) sensors were used and added metal-organic frameworks (MOFs) to make VOC detection possible. MOFs are known for being stable, diverse, and good at grabbing onto certain chemicals like VOCs, making them a good fit for the sensors.
The project started by examining and comparing different ways to sense VOCs. It then focused on why FO-SPR sensors are promising for detecting VOCs and explaining how these work. It also looked closely at MOFs, talking about their structure and how they work, especially in combination with FO-SPR sensors.
All this knowledge was used in a study to find VOCs using FO-SPR sensors modified with MOFs. The study successfully found different types of alcohols at certain levels, showing that MOF-modified FO-SPR sensors can really find VOCs.
Although this first study worked well, it was realized that the sensor could be used even better by looking at the full range of light the FO-SPR sensor produces. So, a new model was created to analyze the entire spectrum of light, providing more information about the substances detected by the sensors. The new model was demonstrated by determining the thickness and refractive index of the MOF in real-time when it was applied to the FO-SPR sensor.
After closely examining these results and the created model, it was observed that things could be improved even further. Thus, another more extensive model was developed that more accurately simulates the FO-SPR sensor. This new model uses three-dimensional ray-tracing and can, as a result, simulate rays at more angles. This makes this model a more versatile tool for further development of these sensors than the old model.
In short, new FO-SPR sensors were successfully created that use MOFs to detect VOCs selectively and sensitively. Also, a new model was created to fully leverage the capabilities of these sensors.
https://lirias.kuleuven.be/handle/20.500.12942/732068







PHD Defence Harshita Bhatia

The synthesis parameters of organic-inorganic lead halide perovskites (OIHPs) will be extensively modulated in order to obtain a whole variety of crystal sizes and morphologies. After profound structural and photophysical characterizations, the performance of differently shaped perovskites in light emitting devices (LEDs) will be investigated. We hypothesize that diverse sizes and morphologies can exhibit different conductive properties when employed as an emissive layer in a multi-layered structure of an LED. Furthermore, composites of emissive perovskites with conductive polymers have a high potential to enhance the optoelectronic properties and carrier mobility in the devices.
https://lirias.kuleuven.be/retrieve/731125
PHD Defence Li Sun

Metal clusters (MCLs), stable groupings of a few to hundreds of metal atoms, have gained significant attention due to their unique electronic characteristics and versatile applications. However, the tendency of individual metal atoms to cluster and form larger nanoparticles diminishes their desirable properties. To counteract this, zeolites, rigid frameworks of aluminosilicate, have emerged as promising hosts. They effectively incorporate metal ions through ion exchange, facilitating MCL formation using diverse approaches. The optical features of MCLs can be tuned by different factors, and the understanding of their structure-property relationship will lay the groundwork for designing MCLs-zeolite materials with specific functions.
In this dissertation, coinage MCLs, including monometallic AgCLs and CuCLs, along with bimetallic AgCuCLs, are encapsulated within LTA and FAU Y zeolites. Upon excitation, these MCLs emit visible light with significant Stokes shifts, high photoluminescent quantum yields, and micro or submillisecond luminescence decay times. Selective synthesis strategies were applied to generate the clusters and optimize their optical properties. Various factors on the photophysical properties of the clusters were discussed systematically. A range of characterization techniques was used to elucidate the structure and formation mechanism of the clusters, as well as their steady-state and excited-state luminescence. An innovative application based on MCLs-zeolite was also validated and thoroughly studied.
In summary, this research contributes valuable insights into the systematic design, photophysical properties, and novel applications of MCLs-zeolite materials. These findings could inspire further exploration of other metal cluster-framework-based materials for diverse applications in imaging, sensing, and optoelectronics.
PHD Defence Lotte Clinckemalie

On Wednesday 6th of September, in the aula of the Arenberg Castle, Lotte had her public PhD defence: "Development of High-Quality Perovskite Materials for Efficient and Stable Radiation Detection"
X-ray imaging is experiencing a significant growing demand in medical diagnostics, resulting in an expected market growth up to €3.8 Bn by 2027. However, the rise in X-ray imaging has led to more frequent exposure of harmful radiation to the human body, which in turn increases radiation-related health risks. To minimize the necessary X-ray exposure for obtaining an image, extensive research is being performed to develop more sensitive X-ray detectors. In the quest for more sensitive materials to reduce the operating radiation dose, metal halide perovskites (MHPs) hold significant potential due to their unique physicochemical properties. However, they still face significant challenges that hinder their commercial availability. One of the main obstacles is the long-term stability of the material. The most popular Pb-based MHPs can undergo phase transitions or complete degradation in response to environmental changes, resulting in a loss of the desirable optoelectronic properties. Another challenge is the deposition of large-area MHP layers for device applications, as conventional fabrication methods typically result in films with a high defect density. Finally, the eco-toxicity of lead-containing perovskites is cited as hampering their future implementation in realistic devices. A viable alternative to the lead halide perovskites are the so called double perovskites, as the corresponding single crystals have proven to generate sensitive and stable X-ray detectors. However, the synthesis of high-quality nano- or microcrystals need to be developed for their implementation in large-area devices. This PhD dissertation presents several promising pathways to address these challenges. Stable, phase-pure, highly crystalline lead-based and lead-free perovskites with excellent photonic properties have been successfully synthesized, characterized and investigated for their potential application as radiation detectors. In Chapter 1, an introduction on CsPbBr3 perovskite single crystals, microcrystals, nanowires, thin films, and nanocrystals was provided, highlighting the importance and challenges of diverse synthesis strategies of perovskite materials on the final performance as photo- and X-ray detectors. In Chapter 3, a new scalable fabrication method of compact, thick, and pinhole-free CsPbBr3 films was presented. Furthermore, we investigated how the implementation of the 2D CsPb2Br5 phase into CsPbBr3 microcrystals affects their structural and optical properties. It was found that the dual-phase sample demonstrates improved tolerance to higher voltage X-ray detection as it exhibits a much lower dark current, linked to suppressed ion migration. The demonstrated low dark current in combination with the plausible formation of shallow defect states, prevent the building up of polarization in the device configuration. This increased stability to higher voltage regimes provides a promising avenue for future improvements in fast, local charge extraction and X-ray image resolution. In Chapter 4, chemical vapor deposition was applied as an advanced synthesis strategy to fabricate smooth, high-quality and phase-pure CsPbBr3 thin films. The impact of the grain size on the carrier transport properties has also been examined, indicating an enhanced mobility upon increased grain size. Additionally, Rb-doping was performed as a strategy to boost the performance of the films as the photoactive layer in photodetectors. Finally, the insights gained from this research was utilized to optimize a home-built setup for the fabrication of CsPbBr3 thin films, with the aim of expanding this strategy towards other (lead-free) perovskite materials in the future. In Chapter 5, the potential application of lead-free perovskite Cs2AgBiBr6 microcrystals in the field of photodetection was highlighted. Cation exchange transformation was suggested as a synthesis method to fabricate microcrystals of higher quality compared to crystals fabricated by direct synthetic routes. To gain insights in the cation exchange process, the reaction mechanism from Cs3Bi2Br9 to Cs2AgBiBr6 has been studied to understand the crystal phase evolution as well as the changes in structural and optical properties of the materials at different stages of the reaction. Furthermore, a proof-of-concept photodetector device indicated that the Cs2AgBiBr6 microcrystals obtained by cation exchange exhibit an improved photoresponse when compared to those obtained by the direct antisolvent precipitation synthesis method. This crystallographic transformation may pave pathway for the development of difficult-to-access perovskite structures, which are not available via direct solution-based synthesis pathways. Overall, the findings presented in this PhD dissertation contribute to the advancement of Pb-based and lead-free MHP materials for radiation detection. Three promising synthesis methods were explored and optimized to address the challenges related to the material stability of MHPs, the development of large-area pinhole-free films for device fabrication, and the lead toxicity. This PhD dissertation provides valuable insights obtained via advanced structural and optical studies and indicates the potential of perovskites as low- and high-energy photodetectors in the future.
PHD Defence Michaël Gebruers (Monday the 21st of August)

PhD defence of Dr. Michaël Gebruers, titled :
Development of allotropic noble metal nanoparticles for improved catalysis
Noble metal nanoparticles (NPs) are often used as a catalyst due to their favorable surface-to-volume ratio and their unique properties, such as the surface activity, compared to their bulk counterparts. The influence of NP size and shape on the surface activity has been widely explored, the influence of the crystal phase has however gained much less attention. In recent years, allotropic NPs of various metals have successfully been obtained. Many of these synthesis however yield only very small amounts of material. This Ph.D thesis aimed to generate scalable, colloidal synthesis of allotropic Ag and Ru NPs, and the evaluation of these materials under catalytic conditions. Chapter 1 summarizes the basic concepts of allotropy and polymorphism in general and specifically applied on noble metal NPs. The synthesis, properties and stability of allotropic Ag and Ru are discussed in detail. Ag and Ru were studied in this work since both metals are known to occur in allotropic structures, functioning as an ideal starting point for the optimization of the synthesis of their allotropic structure. Ag normally occurs in the face-centered cubic (fcc) structure under ambient conditions. The synthesis of allotropic Ag microparticles is not yet fully optimized and usually leads to the formation of mixed structures with only small amounts of hexagonally close-packed (hcp) Ag. Ru normally occurs in the hcp structure under ambient conditions, but can also be synthesized in the allotropic fcc structure. The synthesis of phase pure allotropic Ru NPs is well documented. Finally, the current state of the art regarding the characterization of (allotropic) metal nano- and microparticles is thoroughly discussed to function as a starting point for their further development. The colloidal synthesis of hcp Ag microparticles is investigated and optimized in Chapter 2. First, an improved evaluation of the obtained crystal phase based on X-ray diffraction (XRD) is developed as the peak assignment reported in literature is ambiguous. This new peak assignment was confirmed via XRD simulations based on our experimental data and the space group of hcp Ag. Next, the colloidal synthesis of hcp Ag was optimized by fine-tuning the synthesis parameters and the addition of surfactants, demonstrating the importance of controlling both the surface energy of the developing NPs and the reaction kinetics in the synthesis of allotropic noble metals. While pure hcp Ag could not be obtained, the improved synthesis yields a hcp fraction of 0.71. Finally, the stability of the newly formed hcp Ag microparticles was studied in various solvents, under increased temperature and under increased N2, O2 and H2 pressures. These results outline catalytic conditions under which hcp Ag could be used as catalyst. Chapter 3 studies the effect of both regular hcp and allotropic fcc Ru NPs on the photocatalytic activity of TiO2 in the simultaneous oxidation of benzyl alcohol and H2 production. Loading Ru NPs on TiO2 causes the formation of a Schottky barrier at the Ru/TiO2 interface, resulting in an increased charge separation and a retarded charge carrier recombination. Catalytic experiments showed a ca. 4 and ca. 8 fold increased catalytic activity when loading TiO2 with hcp and fcc Ru NPs respectively, compared to bare TiO2. These increased activities are in line with the measured Schottky barrier heights, for hcp and fcc Ru/TiO2, which were respectively 0.3 and 1.2 eV. The increased Schottky barrier of fcc Ru/TiO2, compared hcp Ru/TiO2, is a result of the strongly increased work function of fcc Ru NPs, compared to hcp Ru NPs. The analysis of the Schottky barrier height, the hcp and fcc Ru NP work function, and the catalytic activity are all in agreement with each other. The properties of fcc and hcp Ru NPs, loaded on TiO2, as thermal catalysts was studied in Chapter 4. The selective hydrogenation of cinnamaldehyde was used as a model reaction since all three of its hydrogenation products, 3-phenylpropanol, 3-phenylpropanal and cinnamyl alcohol, are industrially valuable intermediates. Three different sets of reaction conditions, each resulting in a high conversion and selectivity for one of the three products, were proposed. These reaction conditions were reached by tuning the reaction temperature, the H2 pressure, the solvent and the crystal phase of the Ru NPs. It was demonstrated via DFT simulations that the fcc Ru NPs have a strongly increased work function, compared to the hcp Ru NPs. This increased work function indicates a higher surface stability for the fcc Ru NPs, explaining the decreased catalytic activity of fcc Ru NPs, compared to hcp Ru NPs, in the thermal catalysis of cinnamaldehyde. In conclusion, my Ph.D. work focused on the development and application of allotropic metal particles as catalyst materials. In this work it was demonstrated that the synthesis of allotropic metal particles is not straightforward and that specific methods should be developed for the synthesis of allotropic structures of different metals, since there is no single method that can be employed for all types of metals. The stability of allotropic Ag and Ru was studied, showing that these materials have a good stability range, but that the limitations of their stability (e.g. temperature, pressure or solvent stability) should be taken into account when selecting the catalytic applications of these materials. Finally, it was shown that different allotropic structures of a material perform different in different catalytic applications, it can therefore be concluded that there is not one better catalyst when comparing metal allotropes, but that the best performing allotrope will strongly dependent on the catalytic reaction at hand.
Advanced optical detection of carbon nanoparticles

The air we breathe is full of microscopic particles. This particulate matter is very complex, both in size and composition. Different components of particulate matter contain metal oxides, silica dust, and soot particles. Soot particles are a major form of air pollution in the industrialized world. Every combustion engine that runs, barbecues and campfires that are lit, and unwanted (natural) fires release huge amounts of soot particles into the atmosphere.
Among various components of particulate matter, soot particles, such as 'black carbon' (black carbon, BC) and 'brown carbon' (brown carbon, BrC), not only make a very significant contribution to climate change, but there is also evidence that they have a negative impact on human and animal health. According to the Flemish Environment Agency, the average particulate matter concentration in Flanders is around 20 µg/m3 (2020), of which approximately 5-10% consists of soot particles. Our respiratory exposure to soot can be estimated at approximately hundreds of soot particles every minute! There is in fact more and more circumstantial evidence that these soot particles from the lungs are distributed throughout the body via the bloodstream. In contrast to the routine measurement of soot particles in (polluted) air, it is very challenging to specifically detect these small carbon nanoparticles, typically smaller than 1 µm, in (carbon-rich) biological material.
The main aim of this thesis included the development of novel linear and non-linear optical techniques for the qualitative and quantitative detection of soot particles in biological samples. In collaboration with Hasselt University, advanced laser techniques were developed that can specifically illuminate soot particles in biological samples and thereby generate a detectable signal from these soot particles. Specifically, this thesis shows that carbon nanoparticles emit a very unique, intense, and visible light signal when irradiated with near-infrared light that is invisible to us. In addition, this technique was further developed in combination with microfluidics to determine the size of carbon nanoparticles in (bio) fluid samples, e.g. human urine samples. It was further shown that different types of soot particles, e.g. BC and BrC, also exhibit a measurably different signal that can be distinguished using different color filters. Based on these findings in my thesis, it is now possible to locate, identify, and discriminate soot particles in biological samples, e.g. human biopsies. These results are a step towards the development of diagnostic tools that can allow a better understanding of the exact toxic effects of small carbon particles in human body.
Full text : https://lirias.kuleuven.be/handle/20.500.12942/703015
Metal Halide Perovskites for Photocatalytic Solar-to-Chemical Energy Conversion
The richness and complexity of physical and chemical processes in perovskites make correlative investigations necessary for a comprehensive understanding on perovskites and their optoelectronic devices. That includes surface, morphology, chemical composition, photoluminescence, electroluminescence, electrical responses and so on. A major drawback of most perovskite materials, however, lays in their instability under operating conditions, which is not a secret. Next to the great importance of surface engineering to in stabilizing perovskites, it is also beneficial in improving their physical properties.Besides the importance of surfaces and interfaces, crystal morphology (in particular at the nanoscale) and local stoichiometry also greatly influence the material properties. By doing the work on surface and interface engineering for perovskite optoelectronic nanomaterials, revenues in stability improvements and in property engineering can be envisaged. Full text : https://lirias.kuleuven.be/handle/20.500.12942/700654
THERMOPLASTIC ELASTOMER MICROFLUIDIC DEVICES FOR BIOLOGY AND CHEMISTRY - (Dissertation Alexander McMillan 2021)
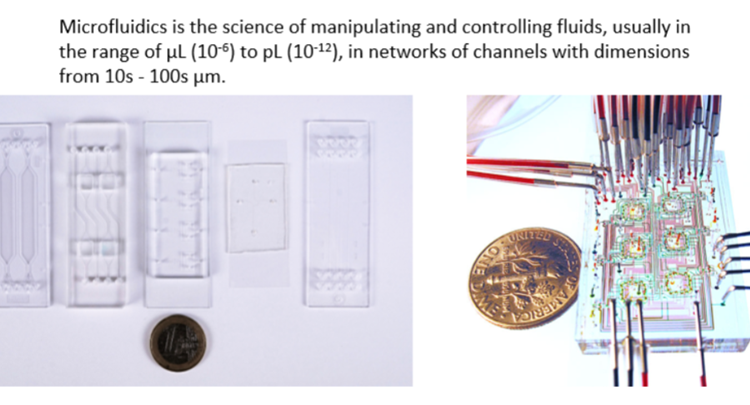
Since its emergence, microfluidics has proven to be a powerful tool in chemistry and the life sciences. Microfluidic devices, consisting of networks of micron-scale flow channels, leverage high surface-area-to-volume ratios and precision fluid control to provide advantages over conventional methods in chemistry and biology. In chemistry, reactions with greater speed, selectivity, and safety can be achieved thanks to fast mixing and efficient heat transfer. In biology, greater control over mechanical and biochemical microenvironments allow cell culture studies with greater relevance to living organisms.
The progression of microfluidics over the past three decades, however, has not lived up to the high expectations that were held at its beginnings. While numerous factors can be identified as bottlenecks in the continued development of microfluidics, one critical element is the need for new microfluidic materials. Microfluidic devices, or “chips,” can be fabricated from a variety of different materials, such as silicon, glass, and polymers, with each one possessing its intrinsic advantages and drawbacks. A material must possess suitable material properties for the microfluidic application at hand, but one must also evaluate its fabrication and cost as factors key to its accessibility and transferability across manufacturing scales. The most common microfluidic material, an elastomer called polydimethylsiloxane (PDMS), possesses numerous drawbacks in its material properties that make it unideal for many biology applications and unusable for many chemistry applications. Moreover, the techniques used for its fabrication are low-throughput, limiting the possibility of large-scale implementation (i.e., a transfer from academic research to industry). A group of materials called soft thermoplastic elastomers (sTPE) have been recently developed for microfluidics, with preliminary reports in literature demonstrating their favorable material properties and transferable fabrication methodologies. This PhD, conducted between academia and industry, focuses on two distinct sTPE materials, Flexdym™ and Fluoroflex, and their use for cell culture and flow chemistry applications, respectively. It aims to evaluate the properties of these novel sTPE materials and capitalize on them by providing sTPE device demonstrations that give scope for broader and more widespread microfluidic applications in these fields.
Full Text : https://lirias.kuleuven.be/handle/123456789/669998
RATIONAL DESIGN OF CATALYST USING ADVANCED OPTICAL MICROSCOPY (Dissertation Guillaume Fleury 2021)

Catalysis forms the cornerstone of the chemical industry, with over 90% of the industrial-scale processes involving at least one catalytic step, mainly carried out using heterogeneous catalysts. These solid materials lower the activation energy of chemical reactions occurring on their surface, enhancing the reaction rate and thus enabling processes at milder operational conditions and with enhanced product selectivity. Furthermore, the material structure's geometrical constraints can strongly influence the nature of the chemicals formed, a feature that can be further exploited to improve the reaction selectivity.
The relevant length scales for the catalytic process taking place at the surface of solid materials span from the angstrom-scale with the molecular bond breaking and formation at the active sites to the size of catalyst particles (nm-μm) and catalyst bodies (mm-cm) used in industrial reactors (m). Most of the actual catalyst bodies used in chemical processes are complex agglomerates consisting of the catalytically active solid held together and shaped with binder materials. The catalyst particles are rarely uniform and often feature local structural and compositional variations. While typical catalyst characterization aims to generate averaged pictures generated through bulk techniques looking at the gram or milligram scale, microscopy methodologies allow identifying material heterogeneities. Shedding light on local structural and compositional variability and understanding their origin enables rationalizing the catalytic performance.
Vibrational spectroscopy offers the possibility to target specific molecules of interest without the need of specific probe molecules such as required for fluorescence-based techniques. Spatially resolving the vibrational modes of interest even enables to spatially resolved molecular processes inside a sample using infrared absorption or Raman scattering processes. Spontaneous Raman scattering is an attractive contrast mechanism compared to infrared absorption as it offers a sub-micrometer resolution and 3D sectioning. However, the intrinsically weak signals generated by this phenomenon limit its use in imaging studies. This limitation is overcome by stimulated Raman scattering (SRS) microscopy, an advanced vibrational technique developed in 2008 as imaging contrast. However, although it is a powerful tool, the application of SRS microscopy has been mostly limited to the study of biological samples up to now. In this work, SRS microscopy assays were developed to spatially resolve the fine details of catalytic materials in individual zeolite crystals. In particular, the orientation of templating agents occluded in the pores of a hierarchically intergrown silicalite-1, and the acid properties of mordenite and ZSM-5 were investigated at the sub-micrometer scale using the Raman signature of organic molecules located in their porous structure.
Full Text : https://lirias.kuleuven.be/handle/123456789/668906



METAL HALIDE PEROVSKITES FOR PHOTOCATALYTIC ORGANIC SYNTHESIS
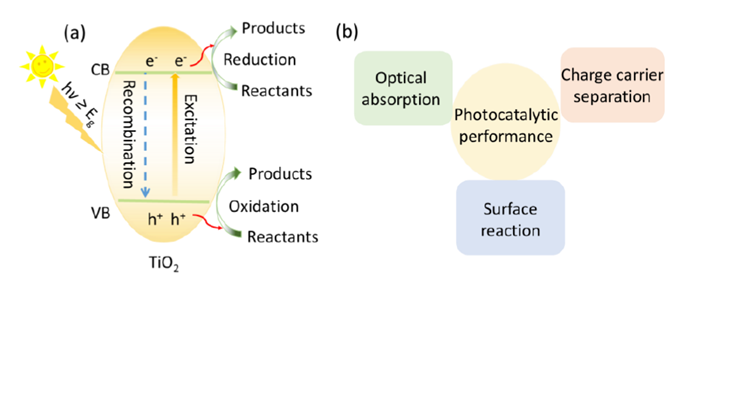
Since Fujishima and Honda first reported in 1972 the photoelectrochemical water splitting of water through TiO2 under UV light irradiation, photocatalysis has attracted a growing amount of research attention. Various types of materials with photocatalytic properties such as metal oxides, metal sulfides, polymers, MOFs etc have been reported for solar energy conversion, storage and utilization, through redution and/or oxidation reactions generating added value products including hydrogen, oxygen and hydrocarbons. Recently, metal halide perovskites(MHPs) have found tremendous popularity in solar to electricity conversion due to the high extinction coefficients, the wide absorption range and long electron−hole diffusion lengths of these materials. All of the mentioned MHP properties match well with the necessary conditions set forward for photocatalytic materials making them potentially interesting photocatalyst, a main concern is however related to their (chemical) stability under reaction conditions.
In this study, the concept of photocatalysis and the development of photocatalytic materials in the last decades are discussed. Next, the photoelectric properties of MHPs are highlighted with an emphasis on their potential as cheap and easy to generate photocatalytic material. After that, the issues hampering MHP-based photocatalysis are identified and general approaches to achieve promising and stable photocatalytic reaction environments are pointed out. Further, we detail the measures being taken to arrive at intrinsically stable photocatalytic materials, removing the need for atypical environments. We first report on the utilization of formamidinium lead bromide (FAPbBr3) as photocatalyst for the selective oxidation of benzylic alcohols to corresponding aldehydes in toluene. To further improve the photocatalytic activity of FAPbBr3, a hybrid material with TiO2, FAPbBr3/TiO2, was prepared by the in-situ anti-solvent growth. The TiO2 extract the photo-generated electrons from FAPbBr3 reducing the charge carrier recombination and enhancing the relative photocatalytic efficiency. Then, the scope of selective chemical conversions that can be photocatalysed with MHPs is expanded. FAPbBr3 was used for the more challenging C(sp3)-H activation in alkanes. Inspired by MHPs solar cell structure, the addition of an electron transfer layer (TiO2) as well as an hole transfer layer (NiOx) allows for further optimization of the conversion efficiency, by further improving the charge separation properties. This TiO2/FAPbBr3/NiOx construction achieved high conversions of C(sp3)-H bond in alkanes to form aldehydes with excellent selectivities. However, band alignment in the FAPbBr3/TiO2 and NiOx/FAPbBr3/TiO2 composites further decreases the redox ability. At last, a perovskite-based direct Z-scheme photocatalyst, consisting of FAPbBr3 and Bi2WO6, is generated for efficient artificial photosynthesis. To maximally utilize the gained redox ability of the Z-scheme photocatalyst, the CO2 reduction is coupled to the benzyl alcohol oxidation.
Overall, in this study metal halide perovskites, with FAPbBr3 as prime example, were used to drive organic reactions through visible light photocatalysis. Type II heterojunctions, including single junction and dual-junctions, were successfully generated to optimize charge carrier separation and transportation yielding a strongly improved photo-activity. Next, a direct Z-scheme photocatalyst with strong redox ability, consisting of FAPbBr3 and Bi2WO6, was used to drive organic synthesis coupled with CO2 reduction. Overall, this work opens a new window for applying MHPs photocatalysis in organic synthesis and also proposes some strategies to improve the activity.
Full Text : https://lirias.kuleuven.be/handle/123456789/653397