Roeffaers Lab
Nanoscopy and catalysis
phd defence
THERMOPLASTIC ELASTOMER MICROFLUIDIC DEVICES FOR BIOLOGY AND CHEMISTRY - (Dissertation Alexander McMillan 2021)
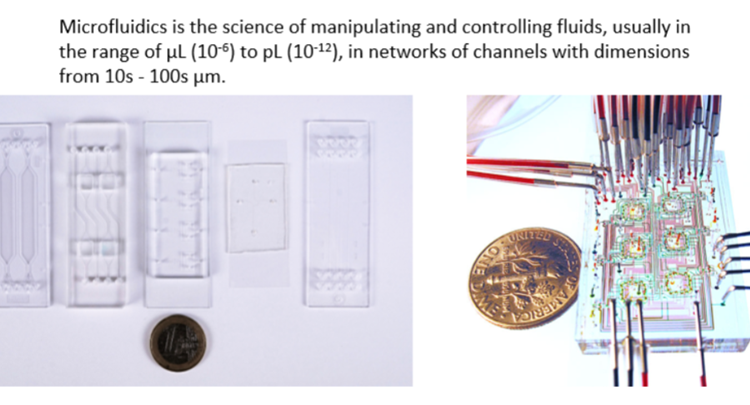
Since its emergence, microfluidics has proven to be a powerful tool in chemistry and the life sciences. Microfluidic devices, consisting of networks of micron-scale flow channels, leverage high surface-area-to-volume ratios and precision fluid control to provide advantages over conventional methods in chemistry and biology. In chemistry, reactions with greater speed, selectivity, and safety can be achieved thanks to fast mixing and efficient heat transfer. In biology, greater control over mechanical and biochemical microenvironments allow cell culture studies with greater relevance to living organisms.
The progression of microfluidics over the past three decades, however, has not lived up to the high expectations that were held at its beginnings. While numerous factors can be identified as bottlenecks in the continued development of microfluidics, one critical element is the need for new microfluidic materials. Microfluidic devices, or “chips,” can be fabricated from a variety of different materials, such as silicon, glass, and polymers, with each one possessing its intrinsic advantages and drawbacks. A material must possess suitable material properties for the microfluidic application at hand, but one must also evaluate its fabrication and cost as factors key to its accessibility and transferability across manufacturing scales. The most common microfluidic material, an elastomer called polydimethylsiloxane (PDMS), possesses numerous drawbacks in its material properties that make it unideal for many biology applications and unusable for many chemistry applications. Moreover, the techniques used for its fabrication are low-throughput, limiting the possibility of large-scale implementation (i.e., a transfer from academic research to industry). A group of materials called soft thermoplastic elastomers (sTPE) have been recently developed for microfluidics, with preliminary reports in literature demonstrating their favorable material properties and transferable fabrication methodologies. This PhD, conducted between academia and industry, focuses on two distinct sTPE materials, Flexdym™ and Fluoroflex, and their use for cell culture and flow chemistry applications, respectively. It aims to evaluate the properties of these novel sTPE materials and capitalize on them by providing sTPE device demonstrations that give scope for broader and more widespread microfluidic applications in these fields.
Full Text : https://lirias.kuleuven.be/handle/123456789/669998
RATIONAL DESIGN OF CATALYST USING ADVANCED OPTICAL MICROSCOPY (Dissertation Guillaume Fleury 2021)

Catalysis forms the cornerstone of the chemical industry, with over 90% of the industrial-scale processes involving at least one catalytic step, mainly carried out using heterogeneous catalysts. These solid materials lower the activation energy of chemical reactions occurring on their surface, enhancing the reaction rate and thus enabling processes at milder operational conditions and with enhanced product selectivity. Furthermore, the material structure's geometrical constraints can strongly influence the nature of the chemicals formed, a feature that can be further exploited to improve the reaction selectivity.
The relevant length scales for the catalytic process taking place at the surface of solid materials span from the angstrom-scale with the molecular bond breaking and formation at the active sites to the size of catalyst particles (nm-μm) and catalyst bodies (mm-cm) used in industrial reactors (m). Most of the actual catalyst bodies used in chemical processes are complex agglomerates consisting of the catalytically active solid held together and shaped with binder materials. The catalyst particles are rarely uniform and often feature local structural and compositional variations. While typical catalyst characterization aims to generate averaged pictures generated through bulk techniques looking at the gram or milligram scale, microscopy methodologies allow identifying material heterogeneities. Shedding light on local structural and compositional variability and understanding their origin enables rationalizing the catalytic performance.
Vibrational spectroscopy offers the possibility to target specific molecules of interest without the need of specific probe molecules such as required for fluorescence-based techniques. Spatially resolving the vibrational modes of interest even enables to spatially resolved molecular processes inside a sample using infrared absorption or Raman scattering processes. Spontaneous Raman scattering is an attractive contrast mechanism compared to infrared absorption as it offers a sub-micrometer resolution and 3D sectioning. However, the intrinsically weak signals generated by this phenomenon limit its use in imaging studies. This limitation is overcome by stimulated Raman scattering (SRS) microscopy, an advanced vibrational technique developed in 2008 as imaging contrast. However, although it is a powerful tool, the application of SRS microscopy has been mostly limited to the study of biological samples up to now. In this work, SRS microscopy assays were developed to spatially resolve the fine details of catalytic materials in individual zeolite crystals. In particular, the orientation of templating agents occluded in the pores of a hierarchically intergrown silicalite-1, and the acid properties of mordenite and ZSM-5 were investigated at the sub-micrometer scale using the Raman signature of organic molecules located in their porous structure.
Full Text : https://lirias.kuleuven.be/handle/123456789/668906



METAL HALIDE PEROVSKITES FOR PHOTOCATALYTIC ORGANIC SYNTHESIS
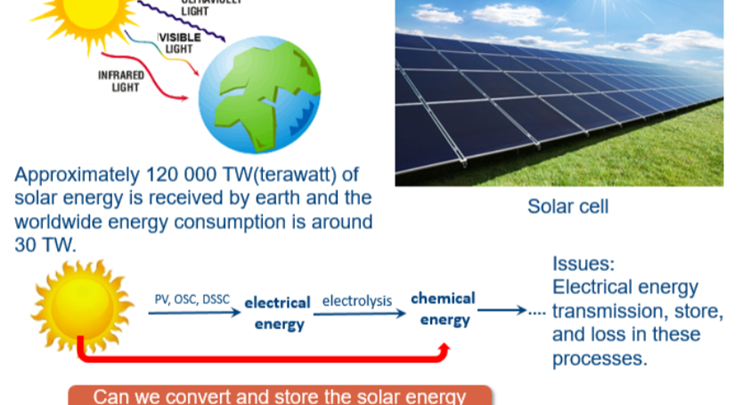
Since Fujishima and Honda first reported in 1972 the photoelectrochemical water splitting of water through TiO2 under UV light irradiation, photocatalysis has attracted a growing amount of research attention. Various types of materials with photocatalytic properties such as metal oxides, metal sulfides, polymers, MOFs etc have been reported for solar energy conversion, storage and utilization, through redution and/or oxidation reactions generating added value products including hydrogen, oxygen and hydrocarbons. Recently, metal halide perovskites(MHPs) have found tremendous popularity in solar to electricity conversion due to the high extinction coefficients, the wide absorption range and long electron−hole diffusion lengths of these materials. All of the mentioned MHP properties match well with the necessary conditions set forward for photocatalytic materials making them potentially interesting photocatalyst, a main concern is however related to their (chemical) stability under reaction conditions.
In this study, the concept of photocatalysis and the development of photocatalytic materials in the last decades are discussed. Next, the photoelectric properties of MHPs are highlighted with an emphasis on their potential as cheap and easy to generate photocatalytic material. After that, the issues hampering MHP-based photocatalysis are identified and general approaches to achieve promising and stable photocatalytic reaction environments are pointed out. Further, we detail the measures being taken to arrive at intrinsically stable photocatalytic materials, removing the need for atypical environments. We first report on the utilization of formamidinium lead bromide (FAPbBr3) as photocatalyst for the selective oxidation of benzylic alcohols to corresponding aldehydes in toluene. To further improve the photocatalytic activity of FAPbBr3, a hybrid material with TiO2, FAPbBr3/TiO2, was prepared by the in-situ anti-solvent growth. The TiO2 extract the photo-generated electrons from FAPbBr3 reducing the charge carrier recombination and enhancing the relative photocatalytic efficiency. Then, the scope of selective chemical conversions that can be photocatalysed with MHPs is expanded. FAPbBr3 was used for the more challenging C(sp3)-H activation in alkanes. Inspired by MHPs solar cell structure, the addition of an electron transfer layer (TiO2) as well as an hole transfer layer (NiOx) allows for further optimization of the conversion efficiency, by further improving the charge separation properties. This TiO2/FAPbBr3/NiOx construction achieved high conversions of C(sp3)-H bond in alkanes to form aldehydes with excellent selectivities. However, band alignment in the FAPbBr3/TiO2 and NiOx/FAPbBr3/TiO2 composites further decreases the redox ability. At last, a perovskite-based direct Z-scheme photocatalyst, consisting of FAPbBr3 and Bi2WO6, is generated for efficient artificial photosynthesis. To maximally utilize the gained redox ability of the Z-scheme photocatalyst, the CO2 reduction is coupled to the benzyl alcohol oxidation.
Overall, in this study metal halide perovskites, with FAPbBr3 as prime example, were used to drive organic reactions through visible light photocatalysis. Type II heterojunctions, including single junction and dual-junctions, were successfully generated to optimize charge carrier separation and transportation yielding a strongly improved photo-activity. Next, a direct Z-scheme photocatalyst with strong redox ability, consisting of FAPbBr3 and Bi2WO6, was used to drive organic synthesis coupled with CO2 reduction. Overall, this work opens a new window for applying MHPs photocatalysis in organic synthesis and also proposes some strategies to improve the activity.
Full Text : https://lirias.kuleuven.be/handle/123456789/653397