Roeffaers Lab
Nanoscopy and catalysis
Article in Catalysis Science & Technology
Catalysis Science & Technology article
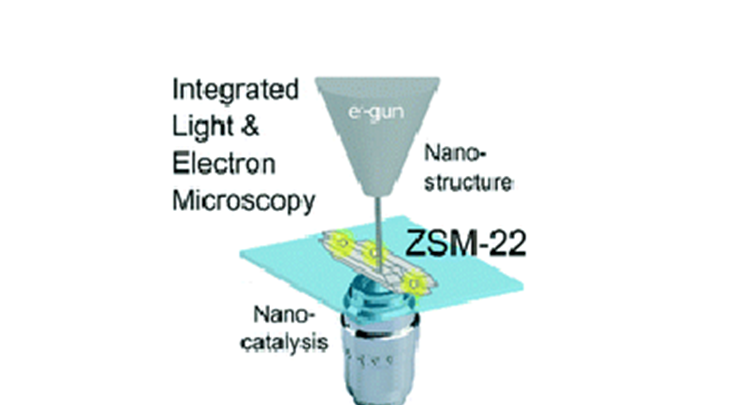
Abstract: Nanocrystalline zeolite particles are applied in a wide range of catalytic reactions. Their size, shape, and the distribution of catalytically active sites significantly vary throughout zeolite batches and within individual particles. This variability leads to a heterogeneous distribution of catalyst performance. Directly investigating the structure–activity relationship at the nanoscale is essential for the rational improvement of catalyst materials. In this work, integrated fluorescence and electron microscopy is employed to correlatively study the structure and performance of individual H-ZSM-22 particles. The needle-shaped morphology of these zeolite particles originates from the lateral fusion of multiple elementary nanorods. Indirect bulk scale experiments have suggested that this process converts catalytically inactive external Al into catalytically active internal Al. The correlative investigation performed in this research provides direct evidence that this conversion takes place, as an inactive shell of 20–40 nm thickness is observed and reactivity is confined to the crystal core. Furthermore, within the catalyst particles nanometer scale catalytic hotspots have been revealed and they are assumed to result from the presence of structural imperfections that locally increase accessibility into the microporous structure. Linear polarized excitation light experiments confirmed that catalytic transformations exclusively occurred on acid sites confined within the microporous structure.
To enable comments sign up for a Disqus account and enter your Disqus shortname in the Articulate node settings.