Roeffaers Lab
Nanoscopy and catalysis
PhD Defence Haowei Huang
METAL HALIDE PEROVSKITES FOR PHOTOCATALYTIC ORGANIC SYNTHESIS
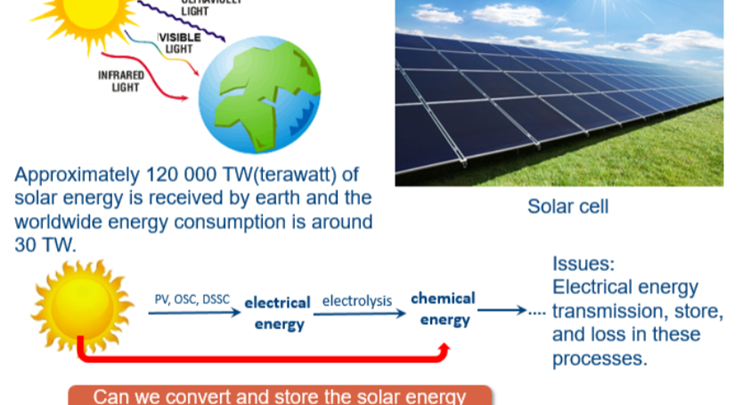
Since Fujishima and Honda first reported in 1972 the photoelectrochemical water splitting of water through TiO2 under UV light irradiation, photocatalysis has attracted a growing amount of research attention. Various types of materials with photocatalytic properties such as metal oxides, metal sulfides, polymers, MOFs etc have been reported for solar energy conversion, storage and utilization, through redution and/or oxidation reactions generating added value products including hydrogen, oxygen and hydrocarbons. Recently, metal halide perovskites(MHPs) have found tremendous popularity in solar to electricity conversion due to the high extinction coefficients, the wide absorption range and long electron−hole diffusion lengths of these materials. All of the mentioned MHP properties match well with the necessary conditions set forward for photocatalytic materials making them potentially interesting photocatalyst, a main concern is however related to their (chemical) stability under reaction conditions.
In this study, the concept of photocatalysis and the development of photocatalytic materials in the last decades are discussed. Next, the photoelectric properties of MHPs are highlighted with an emphasis on their potential as cheap and easy to generate photocatalytic material. After that, the issues hampering MHP-based photocatalysis are identified and general approaches to achieve promising and stable photocatalytic reaction environments are pointed out. Further, we detail the measures being taken to arrive at intrinsically stable photocatalytic materials, removing the need for atypical environments. We first report on the utilization of formamidinium lead bromide (FAPbBr3) as photocatalyst for the selective oxidation of benzylic alcohols to corresponding aldehydes in toluene. To further improve the photocatalytic activity of FAPbBr3, a hybrid material with TiO2, FAPbBr3/TiO2, was prepared by the in-situ anti-solvent growth. The TiO2 extract the photo-generated electrons from FAPbBr3 reducing the charge carrier recombination and enhancing the relative photocatalytic efficiency. Then, the scope of selective chemical conversions that can be photocatalysed with MHPs is expanded. FAPbBr3 was used for the more challenging C(sp3)-H activation in alkanes. Inspired by MHPs solar cell structure, the addition of an electron transfer layer (TiO2) as well as an hole transfer layer (NiOx) allows for further optimization of the conversion efficiency, by further improving the charge separation properties. This TiO2/FAPbBr3/NiOx construction achieved high conversions of C(sp3)-H bond in alkanes to form aldehydes with excellent selectivities. However, band alignment in the FAPbBr3/TiO2 and NiOx/FAPbBr3/TiO2 composites further decreases the redox ability. At last, a perovskite-based direct Z-scheme photocatalyst, consisting of FAPbBr3 and Bi2WO6, is generated for efficient artificial photosynthesis. To maximally utilize the gained redox ability of the Z-scheme photocatalyst, the CO2 reduction is coupled to the benzyl alcohol oxidation.
Overall, in this study metal halide perovskites, with FAPbBr3 as prime example, were used to drive organic reactions through visible light photocatalysis. Type II heterojunctions, including single junction and dual-junctions, were successfully generated to optimize charge carrier separation and transportation yielding a strongly improved photo-activity. Next, a direct Z-scheme photocatalyst with strong redox ability, consisting of FAPbBr3 and Bi2WO6, was used to drive organic synthesis coupled with CO2 reduction. Overall, this work opens a new window for applying MHPs photocatalysis in organic synthesis and also proposes some strategies to improve the activity.
Full Text : https://lirias.kuleuven.be/handle/123456789/653397
To enable comments sign up for a Disqus account and enter your Disqus shortname in the Articulate node settings.